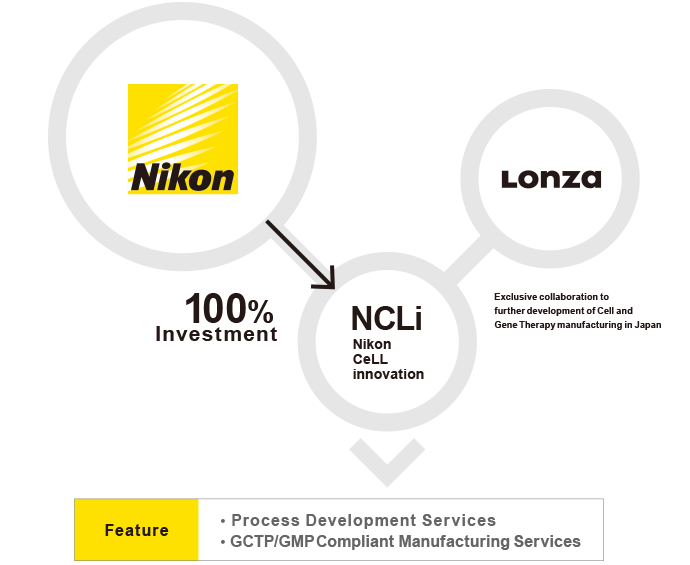
NCLi is well positioned to lead the Japanese regenerative medicine industry in development, and GCTP/GMP* manufacturing services for cell and gene therapies from clinical trial to commercial supply.
Key Capabilities of Lonza
- Experiencesspan
Over two decades of experience in processes development and manufacturing of Cell and Gene Therapies - Proven Performance
Successful delivery of more than 150 clinical and commercial Cell and Gene Therapy projects - Regulatory Support
Longstanding successful collaboration with key regulatory bodies globally - Robust Quality System
Established industry-leading Quality Systems - Innovation
Inventing platform technologies to support manufacturing of commercially viable Cell and Gene Therapies
Global Network
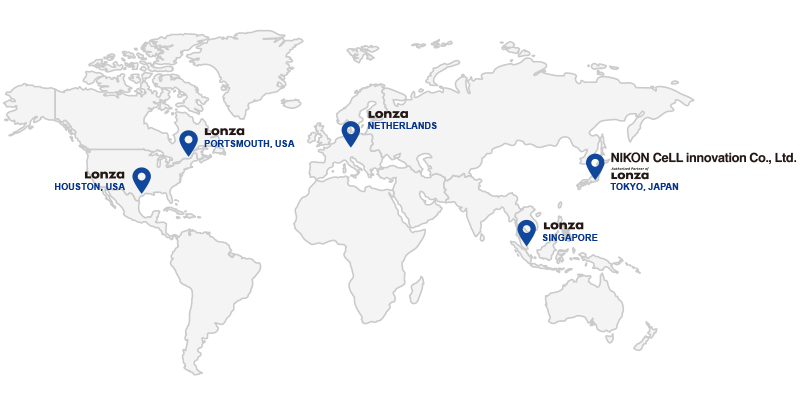
The Nikon-Lonza partnership establishes the NCLi facility as part of Lonza’s overall Cell and Gene Therapy manufacturing network. With similar systems, adapted for local requirements the NCLi facility is established as a sister site to Lonza’s
Houston TX, Portsmouth NH, Tuas Singapore, and Geleen and Maastricht, the Netherlands facilities.
This gives NCLi and Lonza’s customers’ broad geographic reach for manufacturing of their products along with ease of technology transfer
between sites.
- GCTP : Good Gene, Cellular, and Tissue-based Products Manufacturing Practice
- GMP : Good Manufacturing Practice
- CDMO : Contract Development and Manufacturing Organization